Highlight:
UniMoMo is the first all-atom geometric latent diffusion framework capable of designing peptides, antibodies, and small molecules within a unified generative model, demonstrating superior performance and cross-domain knowledge transfer in de novo binder design.
Overview
The design of target-specific binders—ranging from small molecules to peptides and antibodies—is fundamental to modern drug discovery. Historically, computational approaches have treated these molecular types as distinct domains, utilizing specialized generative models for each. This fragmentation fails to leverage cross-domain transferability and limits the exploration of versatile therapeutic strategies. We introduce UniMoMo (Unified generative Modeling of 3D Molecules), a novel framework that unifies the design of diverse molecular binders into a single, cohesive model.
Methodology
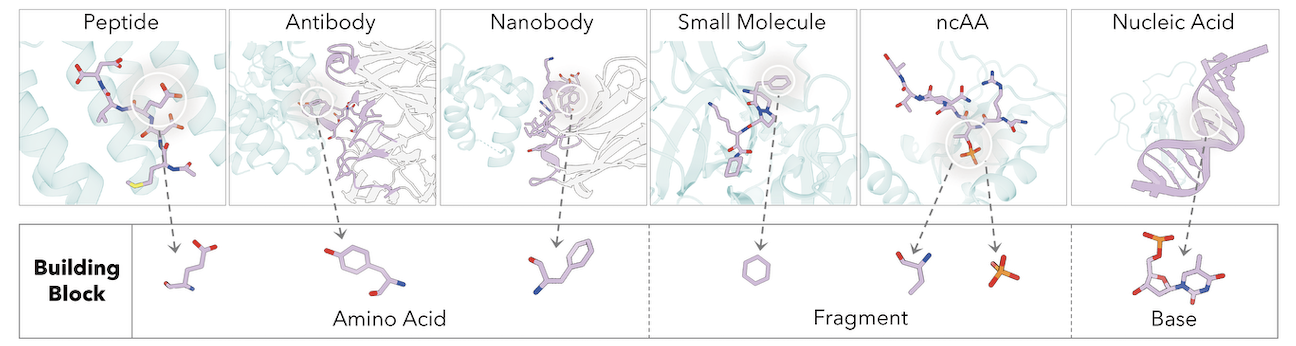
The core innovation of UniMoMo is its ability to process different biomolecular using a unified, full-atom representation called “graphs of blocks.” In this system, a block corresponds to either a standard amino acid (for peptides and antibodies) or a molecular fragment (for small molecules) identified via a principal subgraph algorithm. This representation preserves critical full-atom geometric details while capturing hierarchical structural priors.
To generate new binders, the framework employs a geometric latent diffusion model. The process begins with an iterative full-atom variational autoencoder (VAE), which compresses the “blocks” into a latent space defined by low-dimensional hidden states and spatial coordinates. An E(3)-equivariant diffusion model then operates within this compressed latent space to generate the binder’s structure. This latent-space approach allows the model to focus on global structural arrangements efficiently, while a specialized decoder reconstructs the fine-grained atomic details and chemical bonds.
Performance and Cross-Domain Learning
We benchmarked UniMoMo extensively against state-of-the-art, domain-specific models across peptide, antibody, and small molecule tasks. The unified framework consistently demonstrated superior performance in key metrics, including binding energy and structural validity.
UniMoMo leverages the inherent advantages of multi-domain training within a unified architecture, utilizing cross-domain knowledge transfer to refine all-atom interaction patterns and geometric precision across diverse molecular classes. This approach outperformed variants trained on single domains, indicating that UniMoMo successfully learns and transfers fundamental physical principles governing molecular interactions across different chemical modalities.
Additionally, UniMoMo exbihits highly programmability beyond standard de novo design. By flexibly manipulating 2D topology and 3D geometric “prompt”, the model can tackle specialized tasks such as small molecule fragment growing and linker design. This adaptability also extends to the generation of novel modalities—including cyclic peptides and peptides containing non-canonical amino acids—positioning the model as a versatile solution for a wide array of therapeutic biomolecular design scenarios.
Applications
The versatility of UniMoMo is demonstrated through its ability to generate high-affinity binders across all three modalities for a single target. In a case study targeting KRAS G12D, the model successfully designed peptides, antibodies, and small molecules for the same binding site. In a separate application targeting PCSK9, we leveraged UniMoMo to design small molecules and peptides. The generated candidates exhibited strong binding affinities as confirmed by Surface Plasmon Resonance (SPR). We further characterized AN-5168, a small molecule inhibitor exhibiting strong binding affinity, using Cryo-EM. The analysis revealed high structural fidelity between the resolved structure and the model-predicted binding pose. By bridging the gap between distinct chemical spaces, UniMoMo serves as a powerful tool for holistic drug discovery, enabling the simultaneous exploration of multiple therapeutic modalities.